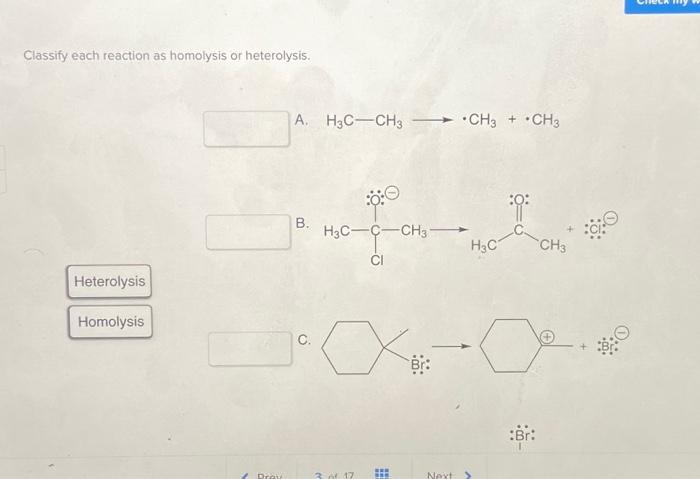
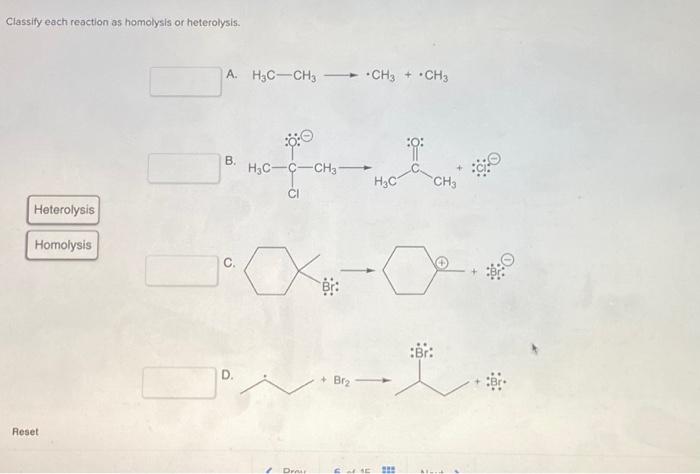
Classify each reaction as homolysis or heterolysis: Homolysis and heterolysis are fundamental concepts in chemistry that describe the two primary mechanisms of bond cleavage. Understanding these mechanisms is crucial for comprehending the reactivity and outcomes of chemical reactions.
This article delves into the intricacies of homolysis and heterolysis, exploring their definitions, differences, and the factors that influence their occurrence. We will also examine the consequences of these bond cleavage mechanisms for reaction products and their applications in organic synthesis.
Homolysis and Heterolysis: Key Concepts
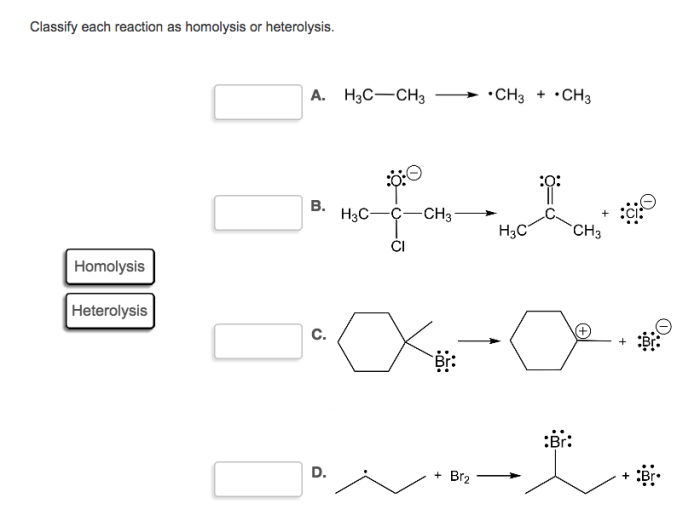
Homolysis and heterolysis are two fundamental types of bond cleavage reactions in chemistry. Understanding their differences is crucial for comprehending reaction mechanisms and predicting product outcomes.
In homolysis, a covalent bond breaks symmetrically, resulting in the formation of two free radicals. Each radical possesses one of the bonding electrons. In contrast, heterolysisinvolves the unsymmetrical cleavage of a covalent bond, leading to the formation of an anion and a cation.
The electron pair from the broken bond is retained by one of the fragments.
The choice between homolysis and heterolysis depends on various factors, including bond strength, electronegativity, and solvent polarity. Generally, homolysis favors weak bonds and nonpolar solvents, while heterolysis favors strong bonds and polar solvents.
Factors Influencing Reaction Type, Classify each reaction as homolysis or heterolysis
The following factors play a significant role in determining whether a reaction proceeds via homolysis or heterolysis:
- Bond Strength:Stronger bonds require more energy to break, making homolysis less favorable. Conversely, weaker bonds favor homolysis.
- Electronegativity:The electronegativity difference between the bonded atoms influences heterolysis. A large electronegativity difference promotes heterolytic cleavage, resulting in the formation of ions with opposite charges.
- Solvent Polarity:Polar solvents stabilize ions formed during heterolysis. Thus, reactions in polar solvents tend to favor heterolysis over homolysis.
Consequences of Homolysis and Heterolysis
The type of bond cleavage has significant consequences for the reaction products:
- Homolysis:Produces free radicals, which are highly reactive and can undergo further reactions, such as radical recombination, disproportionation, or addition to double bonds.
- Heterolysis:Generates ions, which can be stabilized by solvation or undergo nucleophilic or electrophilic reactions. The stability and reactivity of the ions depend on their charge and the surrounding environment.
Applications of Homolysis and Heterolysis
Homolysis and heterolysis are widely employed in organic synthesis to achieve specific transformations:
- Homolysis:Used in radical reactions, such as free radical halogenation, polymerization, and alkylation.
- Heterolysis:Utilized in ionic reactions, including nucleophilic substitution, electrophilic addition, and elimination reactions.
Essential FAQs: Classify Each Reaction As Homolysis Or Heterolysis
What is the key difference between homolysis and heterolysis?
Homolysis involves the symmetrical breaking of a covalent bond, resulting in two radicals, while heterolysis involves the unsymmetrical breaking of a bond, resulting in an anion and a cation.
What factors influence whether a reaction proceeds via homolysis or heterolysis?
Factors such as bond strength, electronegativity, and solvent polarity can influence the type of bond cleavage mechanism.
What are the consequences of homolysis and heterolysis for reaction products?
Homolysis often leads to the formation of free radicals, which can be highly reactive, while heterolysis typically results in the formation of ions, which can be more stable and less reactive.