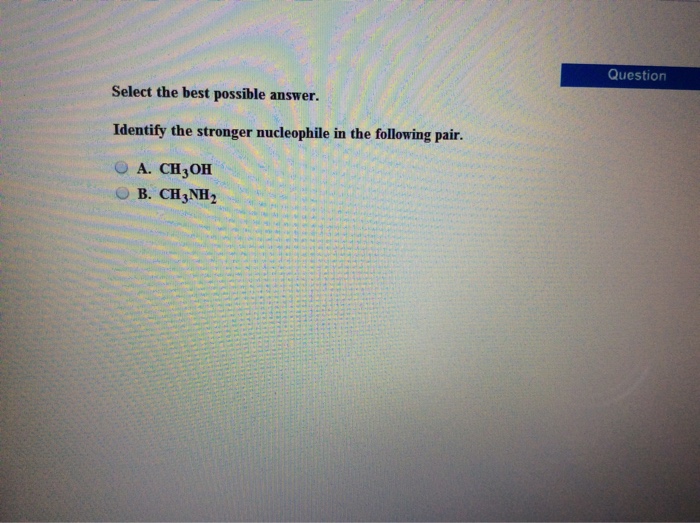
Identify the stronger nucleophile in the following pair. – In the realm of chemical reactions, the identification of stronger nucleophiles is paramount to understanding their behavior and predicting the outcomes of various processes. This guide delves into the concept of nucleophilicity, exploring the factors that influence it and providing a step-by-step approach to identifying the stronger nucleophile in a given pair.
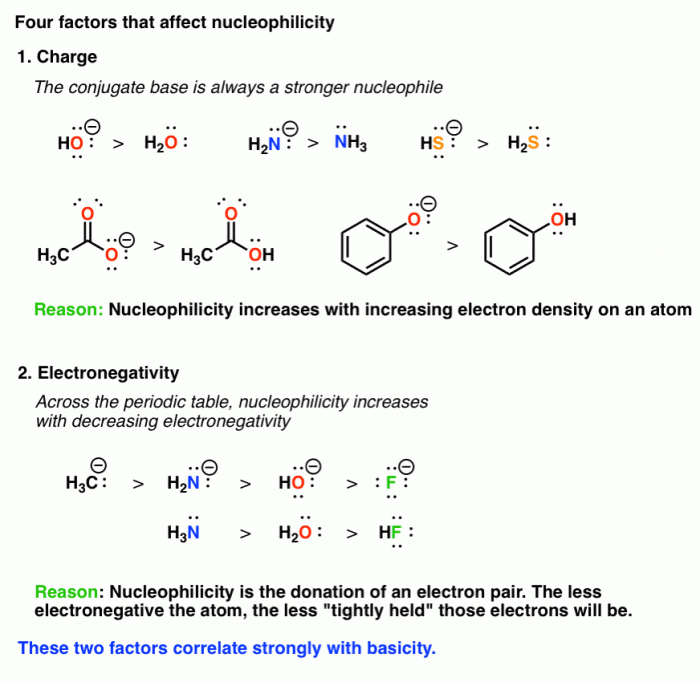
Nucleophiles, electron-rich species with a proclivity to donate electrons, play a crucial role in numerous chemical reactions, including substitution, addition, and elimination reactions. Their strength, known as nucleophilicity, is governed by several key factors, such as charge, size, and polarity.
Identify the Stronger Nucleophile: Identify The Stronger Nucleophile In The Following Pair.
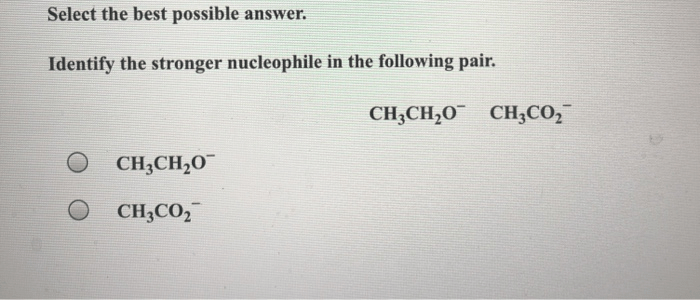
A nucleophile is a chemical species that donates a pair of electrons to form a new chemical bond. Nucleophilicity is the ability of a nucleophile to donate electrons and is influenced by several factors, including charge, size, and polarity.
Factors Affecting Nucleophilicity
- Charge:Nucleophiles with a negative charge are generally stronger than those with a neutral or positive charge.
- Size:Smaller nucleophiles are generally stronger than larger ones.
- Polarity:Nucleophiles with a higher polarity are generally stronger than those with a lower polarity.
Identifying Stronger Nucleophiles, Identify the stronger nucleophile in the following pair.
- Determine the charge of each nucleophile.
- Compare the sizes of the nucleophiles.
- Consider the polarity of the nucleophiles.
- The nucleophile with the most negative charge, smallest size, and highest polarity is generally the stronger nucleophile.
Examples and Counter Examples
- Example:Hydroxide ion (OH –) is a stronger nucleophile than water (H 2O) because it has a negative charge and is smaller.
- Counter Example:Iodide ion (I –) is a weaker nucleophile than bromide ion (Br –) even though it is larger because it has a lower polarity.
Applications of Nucleophilicity
Nucleophilicity plays a crucial role in various chemical reactions, including:
- Substitution reactions:Nucleophiles can replace a leaving group on an electrophile.
- Addition reactions:Nucleophiles can add to an electrophile to form a new bond.
- Elimination reactions:Nucleophiles can abstract a proton from a substrate to form an alkene or alkyne.
FAQ Insights
What is the definition of a nucleophile?
A nucleophile is an electron-rich species that has the ability to donate electrons to an electrophile, forming a new covalent bond.
What factors affect nucleophilicity?
Nucleophilicity is influenced by several factors, including charge, size, and polarity. In general, negatively charged nucleophiles are stronger than neutral or positively charged nucleophiles, smaller nucleophiles are stronger than larger nucleophiles, and more polar nucleophiles are stronger than less polar nucleophiles.